©2022 This excerpt taken from the article of the same name which appeared in ASHRAE Journal, vol. 64, no. 2, February 2022.
About the Authors
Yunho Hwang, Ph.D. is a research professor and co-director of the Center for Environmental Energy Engineering at the University of Maryland, College Park, Md. Ben Greenfield is a director of marketing and business development for Helmer Scientific. Bruce Kranz is an engineering director for Thermo King Americas. Brian Hoaglan is a low temperature products R&D manager for Helmer Scientific. Chris Repice is an engineering fellow for Carrier Corporation. Igor Vaismanis, Ph.D., P.Eng., is a fellow of innovations in thermal management and vapor cycle technologies at Booz Allen Hamilton. Jürgen Olivier is a mechanical engineer at HC Heat Exchangers PTY. Kashif Nawaz, Ph.D., is a senior research scientist at Oak Ridge National Laboratory. Stefan Elbel, Ph.D., is a research assistant professor and associate director, Air Conditioning and Refrigeration Center at University of Illinois, Urbana-Champaign, Ill.
This peer-reviewed article does not represent official ASHRAE guidance. For more information on ASHRAE resources on COVID-19, visit ashrae.org/COVID19.
Differing storage temperature requirements are not the only concern for COVID-19 vaccines. The entire vaccination process—from the original manufacturing to final vaccine administration—involves various refrigerated transportation and storage systems. This article describes various vaccine refrigerated transportation and storage technologies and systems recommendations for the general public and vaccine transport-related professionals.
The article is a product of the ASHRAE Refrigeration Technology Committee for Comfort, Process, and Cold Chain, which formed a group in February 2021 to provide “practical guidance for COVID-19 vaccine refrigerated transportation and storage” to assist stakeholders in the vaccine distribution process from the equipment point of view.
General Recommendations
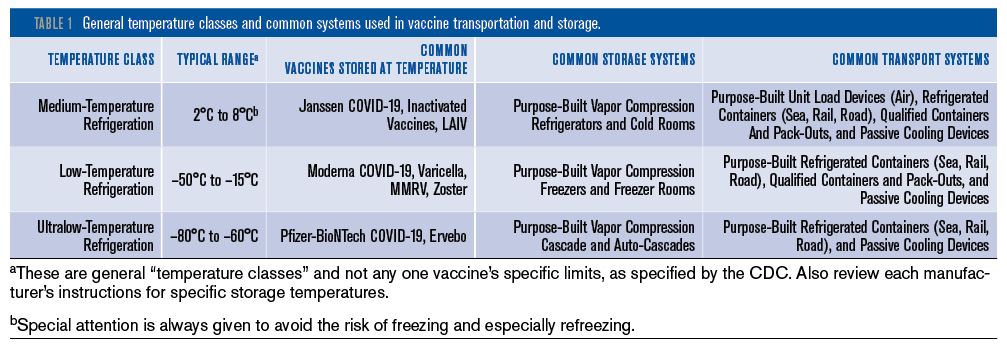
The CDC has produced a Vaccine Storage and Handling Toolkit,1 which provides general recommendations from “minimal actions” to “best practices” for the various steps of the immunization supply chain. Table 1 provides the summary of the general temperature classes and common systems used in vaccine transportation and storage. The range of suitable and purpose-built products essentially spans three distinct temperature bands commonly associated with specific vaccines: medium-temperature refrigeration (2°C to 8°C [35.6°F to 46.4°F]), low-temperature refrigeration (–50°C to –15°C [–58°F to 5°F]), and ultralow-temperature refrigeration (–80°C to –60°C [–112°F to –76°F]).
Vaccines typically also require storage at different temperatures during the different stages of their transportation and handling (although refreezing is strictly prohibited in most instances) and do not usually remain within only one of the temperature bands. Products used for vaccine transportation and storage should, therefore, be both suitable and purpose-built to a pharmaceutical grade. Some of the products listed in the CDC Toolkit as suitable for vaccine transport include refrigerators and freezers, qualified containers and pack-outs, conditioned water bottle transport systems and the manufacturer’s original shipping containers. Products listed as unsuitable include food and beverage coolers for transport (either off-site or emergency), and bar-style refrigerators/freezers for storage.
Notwithstanding references to distinct temperature bands, each vaccine is also defined by its own, unique requirements. This may necessitate tighter temperature control or present stricter allowances for excursions, in addition to the specific temperature constraints placed on the various diluents used alongside vaccines. Given these unique features of each vaccine, the CDC, therefore, also publishes more detailed guidance.
Read the Full Article
ASHRAE Members have free access to the full-text PDF of this article as well as the complete ASHRAE Journal archives back to 1997 in the Free Member Access Area.
Non-members can purchase features from the ASHRAE Bookstore. Or, Join ASHRAE!
Return to Featured Article Excerpts
Return to ASHRAE Journal Featured Article Excerpts